What is Electron Configuration?
Electron configuration is the specific way that electrons are distributed among the atomic orbitals of an atom. The electron configuration of an atom can be determined by using the periodic table. The periodic table is arranged in order of increasing atomic number. This means that the elements are listed in order from left to right and top to bottom on the table. The electron configuration can be found by looking at the element’s box on the table and locating the symbol for that element’s electron configuration. The first column of the periodic table is called the s-block. This column contains elements in which all of the electrons are in s-orbitals. The second column is called the p-block. This column contains elements in which some of the electrons are in p-orbitals. The third column is called the d-block. This column contains elements in which all of the electrons are in d-orbitals. The fourth column is called the f-block. This column contains elements in which some of the electrons are in f-orbitals. The fifth column is called the g-block.
The electron configuration is the standard writing system for describing the electronic structure of an atom. It shows the distribution of electrons in orbitals of an atom or a molecule. For example, the electron configuration of Iron (Fe) is 1s22s22p63s23p64s23d6
How to Make an Electron Configuration Chart?
The electron configuration of an atom is a shorthand notation for representing the arrangement of electrons in an atom. The electron configuration can be written in either orbital notation or Lewis dot notation. In orbital notation, the electrons are listed in order of increasing energy, starting with the lowest-energy orbitals first. In Lewis dot notation, the electrons are represented as dots surrounding the symbol for the element.
The most common way to write the electron configuration is to use orbital notation. To write the electron configuration of an atom using orbital notation, you first need to identify the element’s atomic number. Then, you list the electrons in order of increasing energy, starting with the lowest-energy orbitals first. For example, the electron configuration for oxygen can be written as 1s2 2s2 2p4.
Writing your own electron configuration chart is very easy. You need not look at the chart provided by the periodic table of elements. Sometimes, memorizing some mnemonics of spdf sequence of the chart may be helpful but what if you forget it? Here are the steps on how the electron configuration chart can be written:
- First, memorize the four letters sequence, spdf.
- You need only the numbers 1 to 7.
- On a piece of paper, you need to provide four columns: 1st column (s), 2nd column (p), 3rd column (d), and 4th column (f).
- In the 1st column, write the numbers 1 to 7 downward. In the 2nd column, you need to start writing the numbers 2 to 7 downward in line with the number 2 of the 1st column. In the 3rd column, write the numbers 3 to 7 downward in line with the number 3 of the 2nd column. Lastly, in the 4th column, write the numbers 4 to 7 downward in line with the numbers 4 to 7 of the 3rd column.
- Then, write the letters spdf on their respective columns after each number.
- Finally, write down diagonal arrows pointing downward left starting from 1s of the chart. The diagonal arrows should be parallel to each other. The chart should look like this:
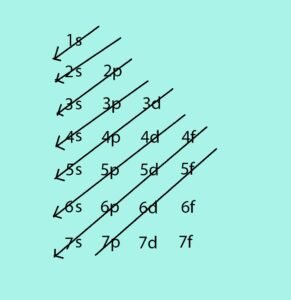
Now, you can write the electron configuration of any element by looking at your electron configuration chart. For example, the electron configuration of Gold(Au) with atomic number of 79 is 1s22s22p63s23p63d104s24p64d104f145s25p65d106s1. Getting the sum of all the superscripts, it will result to 79, which is the atomic number of the element Gold. As you can see, the s orbitals can only hold 2 electrons all throughout the configuration.
| orbitals | electrons |
| s | 2 |
| p | 6 |
| d | 10 |
| f | 14 |
In writing the electron configuration, the correct sequence of the electron configuration chart and considering the holding capacity of each orbital should be followed.



Hello
Spot on with this write-up, I absolutely believe this site needs far more attention. I’ll probably be returning
to read more, thanks for the info!